
Pipeline
Introducing the next generation of disease-modifying therapies
Proprietary Programs
Preclinical
Phase 1
Phase 2
Phase 3
Regulatory Status
CNP-104
Primary Biliary Cholangitis
Preclinical
Phase 1
Phase 2
Phase 3
Regulatory Status:
Orphan Drug & Fast Track Designations
CNP-103
Type 1 Diabetes
Preclinical
Phase 1
Phase 2
Phase 3
Regulatory Status:
Partnered Programs
Preclinical
Phase 1
Phase 2
Phase 3
CNP-104 for primary biliary cholangitis (PBC)
PBC is a chronic autoimmune disease that targets the bile ducts in the liver, leading to progressive bile duct destruction, inflammation, and fibrosis.1 As the disease progresses, fibrosis worsens contributing to patients enduring debilitating symptoms, including cirrhosis, and may ultimately lead to liver failure, the need for a transplant, or death.1 Current therapies for PBC have limited long-term efficacy and fail to address the root cause of the disease.2
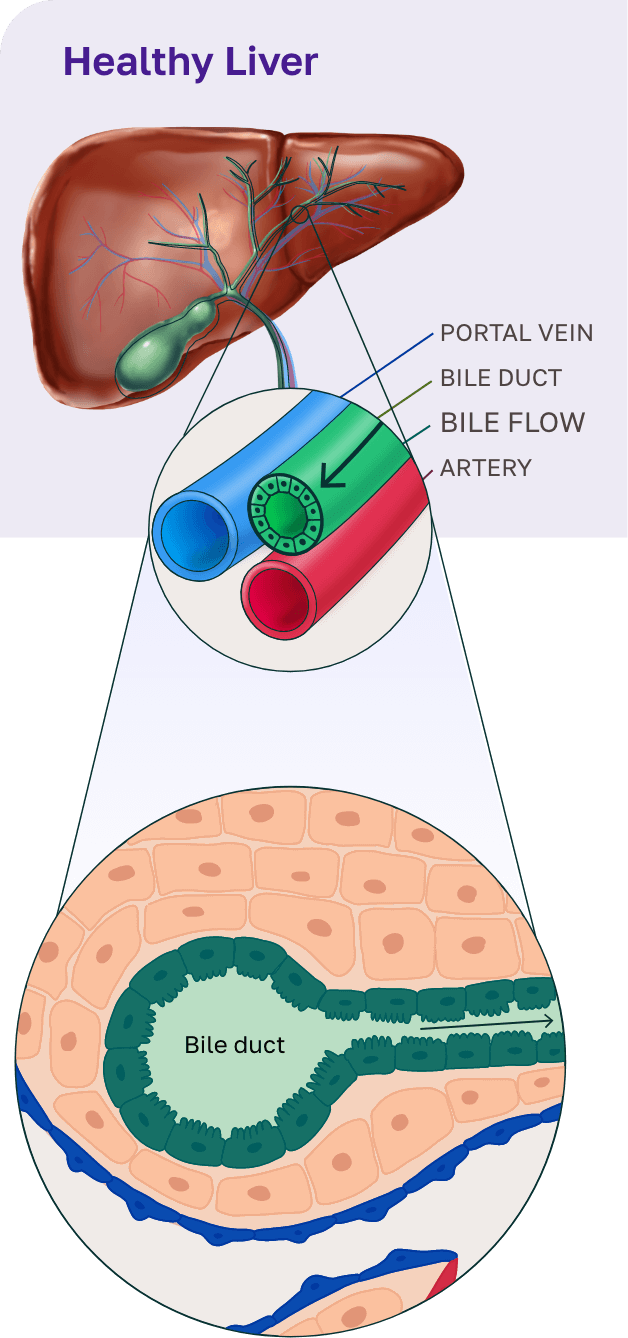
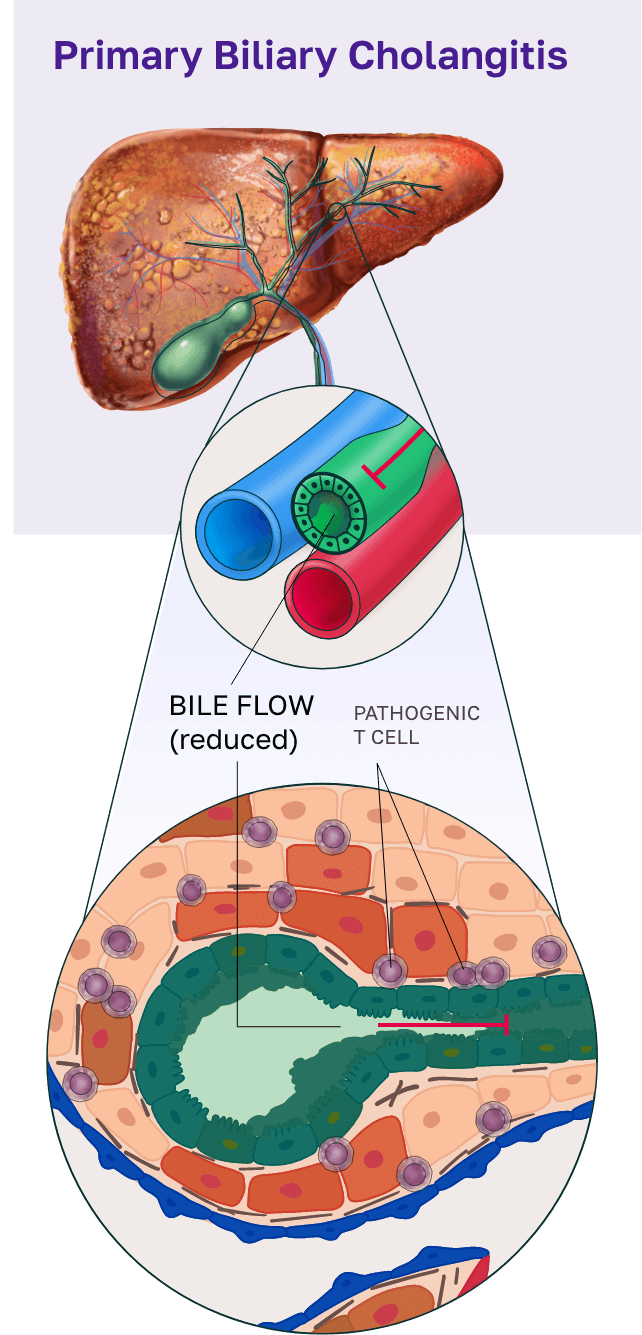
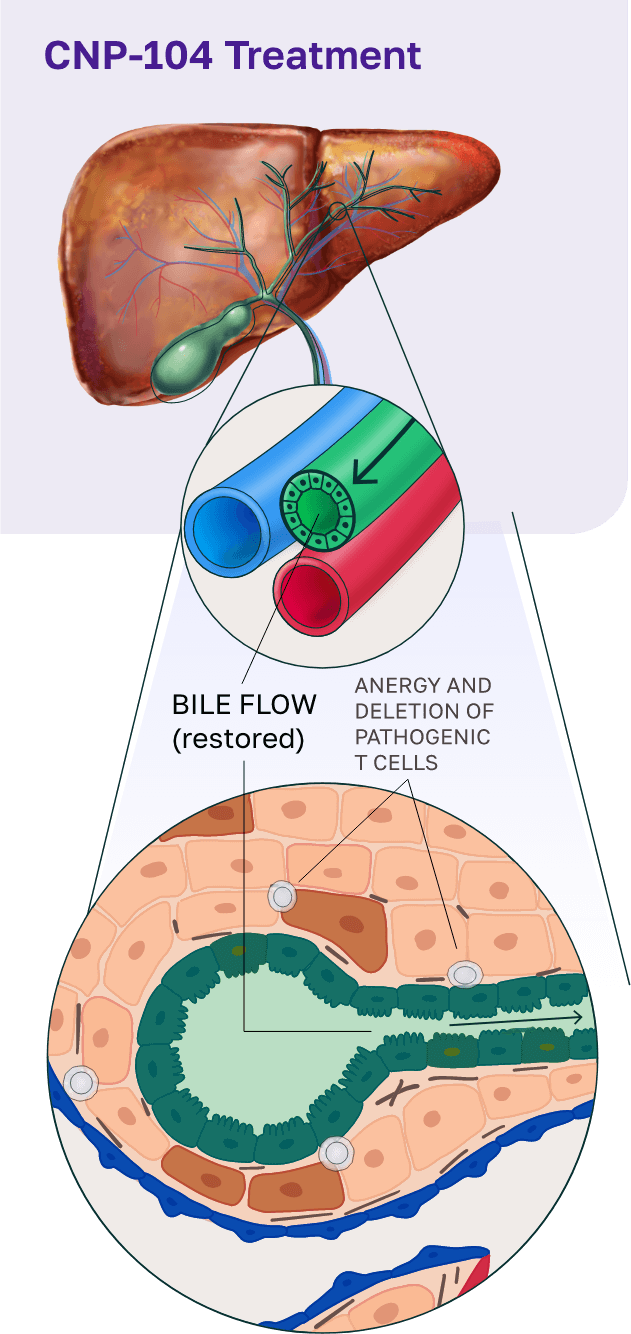
To address this, we developed CNP-104, a first-in-class therapy that targets the root cause of PBC. CNP-104 encapsulates the autoantigen PDC-E2 to reprogram the immune system and specifically regulate pathogenic CD4+ and CD8+ T cells, halting bile duct destruction and disrupting the progression of PBC. This approach offers patients a potential path to restoring normal liver bile duct function and providing lasting disease control.
CNP-104 was granted Fast Track Designation by the United States Food and Drug Administration (FDA) in January 2022 followed by Orphan Drug Designation in December 2024. A phase 2b clinical trial is expected to begin in 2025.
CNP-103 for type 1 diabetes (T1D)
T1D is a progressive disease in which the immune system destroys insulin-producing β cells in the pancreas, leading to lifelong insulin therapy to help regulate blood glucose levels.5 Despite insulin therapy, patients with T1D and their caregivers often live in a state of constant vigilance due to frequent fluctuations in blood glucose levels that can result in hospitalization or death.5,6
While insulin therapy is essential for people with T1D, it does not prevent these severe complications or address the underlying autoimmunity.5 Other therapies that delay disease onset or replace pancreatic islet cells offer partial solutions but do not stop the autoimmune attack or provide lasting β-cell preservation.7
CNP-103 offers a groundbreaking disease-modifying approach by encapsulating 4 recombinant proteins that cover more than 95% of the antigens that drive T1D.
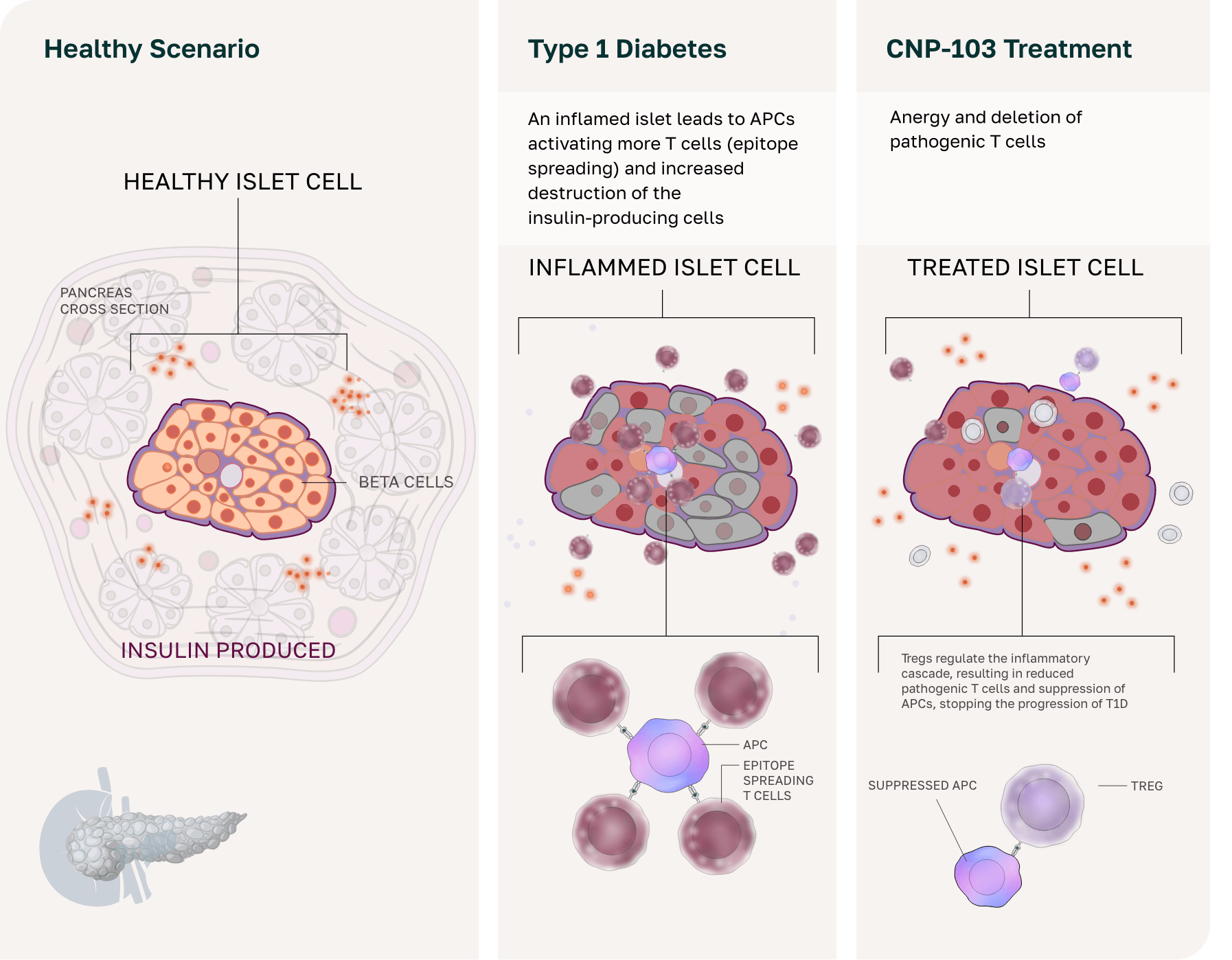
This strategy prevents β-cell destruction by retraining the immune system to tolerate these antigens, enabling natural insulin production maintenance and the potential reversal of glycemic dysregulation.
CNP-103 was granted Fast Track Designation by the United States FDA in March 2025. A first-in-human clinical trial for CNP-103 is planned to begin in 2025.
CNP-106 for myasthenia gravis (MG)
MG is a chronic autoimmune disease that disrupts communication between nerves and muscles, leading to muscle weakness that can dramatically impair basic bodily functions, including vision, speech, swallowing, breathing, and mobility.3 As a result, people living with MG often face severe limitations in daily life and, in some cases, life-threatening crises.3 Current therapies rely on broad immune suppression or antibody depletion but do not target the underlying cause of the disease.3
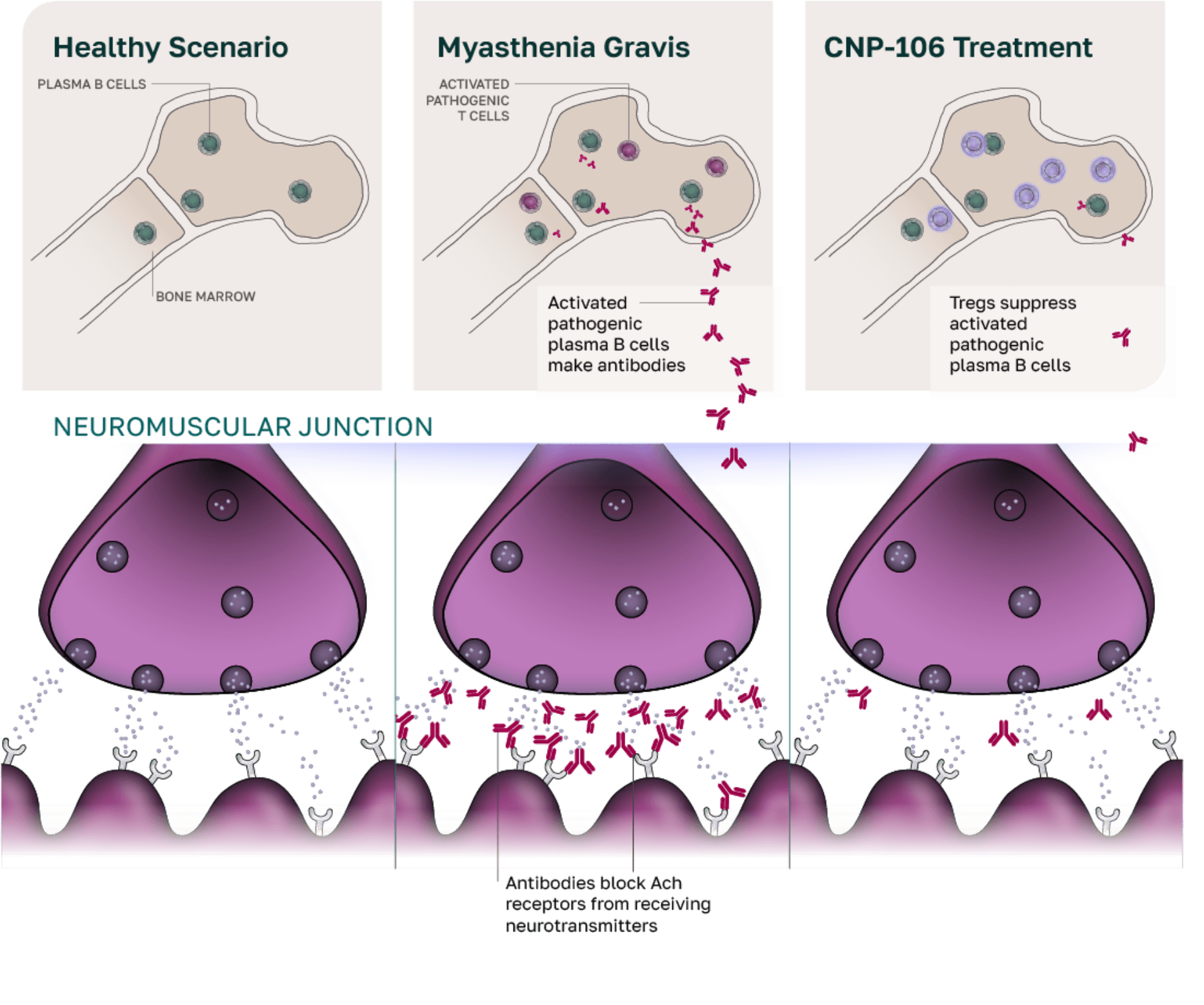
Additional limitations to these treatments include black box warnings, infection risks, complicated dosing protocols, and incomplete treatment response in 30% to 60% of patients.3,4
We aim to overcome these challenges with CNP-106, a breakthrough disease-modifying treatment for MG that encapsulates AChR-associated autoantigens. CNP-106 is designed to reprogram the immune system, resulting in the regulation of AChR antibody-producing B cells, which in turn restores neuromuscular function and provides a targeted and lasting solution for patients with MG.
COUR is enrolling patients with MG in a phase 1b/2a trial to evaluate the safety and efficacy of CNP-106.
CNP-101/TAK-101 for celiac disease
Celiac disease is a serious autoimmune disorder that affects approximately 1% of the global population.8 For patients living with the disease, even small amounts of gluten trigger an immune attack on the small intestine.8 This leads to nutrient malabsorption while increasing the risk of osteoporosis, vitamin deficiencies, and even cancer.8 Following a strict gluten-free diet is the only disease management option, but it does not address the underlying autoimmunity, leaving patients vulnerable to relapse.8

CNP-101/TAK-101 is a first-in-class antigen-specific immune tolerance therapy that is designed to reprogram the immune system to recognize gluten as harmless. In a phase 1b/2a clinical trial, patients treated with CNP-101 before gluten exposure showed significantly reduced immune responses and intestinal destruction compared with those who received placebo. CNP-101 showed potential to protect the intestines from gluten-related injury. Notably, this was the first clinical trial to demonstrate the induction of antigen-specific immune tolerance in humans in any autoimmune disease.
Takeda acquired the license for CNP-101 in 2019 and is enrolling patients in a phase 2b study, paving the way for a future in which celiac disease can be treated at its root cause.
References
- Pandit S, Samant H. Primary Biliary Cholangitis. In: StatPearls [Internet]. StatPearls Publishing; 2025. Updated February 12, 2023. https://www.ncbi.nlm.nih.gov/books/NBK459209/
- Tanaka A. Challenges for diagnosis and treatment of primary biliary cholangitis. In: Rezaei N, ed. Translational Immunology: Challenges for Autoimmune Diseases. 5th ed. Academic Press; 2023:215-241. https://doi.org/10.1016/B978-0-323-85389-7.00007-7
- Lucier J, Mathias PM. Type 1 Diabetes. In: StatPearls [Internet]. StatPearls Publishing; 2025. Updated October 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK507713/
- Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME; DCCT/EDIC Research Group. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care. 2013;36(10):3131-3138. doi:10.2337/dc12-2109
- Suresh AB, Asuncion RMD. Myasthenia Gravis. In: StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 8, 2023. https://www.ncbi.nlm.nih.gov/books/NBK559331/
- Schneider-Gold C, Gilhus NE. Advances and challenges in the treatment of myasthenia gravis. Ther Adv Neurol Disord. 2021;14:17562864211065406. doi:10.1177/17562864211065406
- National Institute of Diabetes and Digestive and Kidney Diseases. Pancreatic islet transplantation. U.S. Department of Health and Human Services; National Institutes of Health. Accessed February 3, 2025. https://www.niddk.nih.gov/health-information/diabetes/overview/insulin-medicines-treatments/pancreatic-islet-transplantation
- National Institute of Diabetes and Digestive and Kidney Diseases. Definition & facts for celiac disease. U.S. Department of Health and Human Services; National Institutes of Health. Accessed February 3, 2025. https://www.niddk.nih.gov/health-information/digestive-diseases/celiac-disease/definition-facts