
First-In-Class Therapies Designed to Reprogram the Immune System

About COUR Who We Are
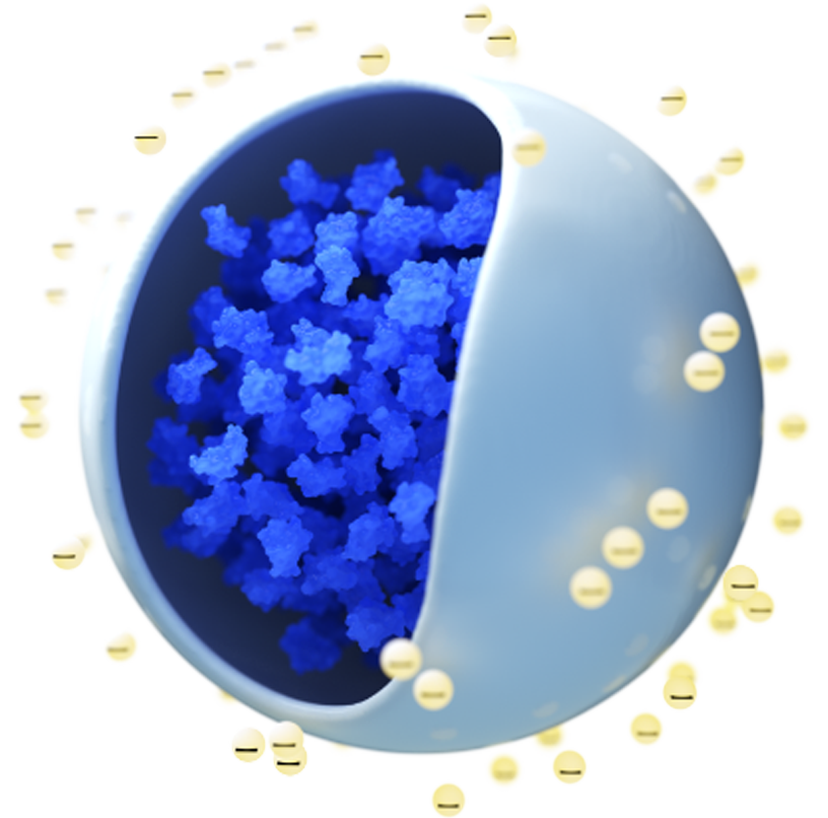
COUR Pharmaceuticals is developing first-in-class therapies designed to reprogram the immune system to achieve antigen-specific tolerance for immune-mediated disease. COUR’s platform of immune-modifying nanoparticles aims to treat the root cause of immune disease, unlike traditional approaches, which only minimize symptoms using toxic immune suppression. COUR’s lead product for celiac disease, partnered with Takeda, is the first demonstration of induction of antigen-specific immune tolerance in any autoimmune disease. Data from clinical and preclinical settings demonstrate the opportunity for the COUR nanoparticle platform to address a wide range of immune and inflammatory conditions.
COUR Nanoparticle Platform Focuses On
Reprogramming the Immune System
Inducing Tolerance to Specific Antigen
Preserving Immune Functionality