
Platform
Autoimmune disease is a global health crisis. We are working to change that.
In the US alone, 23 million people live with autoimmune disease, and as many as 1 in 10 people live with autoimmune disease in the industrial world.1-3 Autoimmune diseases occur when the immune system attacks the body’s own tissues due to molecular mimicry, a process in which self-antigens are misidentified as foreign. This can lead to severe impairments in quality of life, loss of function, and even death.4
Current treatments in autoimmune disease attempt to relieve symptoms of inflammation by indiscriminately targeting the immune system, not addressing the underlying cause, leading to significant collateral risk.4,5 These treatments do not address the underlying condition but rather act to temporarily mask and suppress symptoms of the disease.6 These shortfalls highlight the need for treatments that build and sustain tolerance to multiple disease-driving antigens for a durable and effective response.
Inducing tolerance:
the holy grail of autoimmune disease treatment
At COUR, we are transforming the treatment of autoimmune diseases by addressing the root cause: an immune system that has lost the ability to recognize itself. Our approach tackles the complexity of autoimmunity at its source, reprogramming upstream to target as many antigens as necessary. This includes not only the initiating antigen but also those that may be targeted by epitope spreading after disease onset, ultimately promoting lasting immune tolerance.
The COUR Nanoparticle Platform
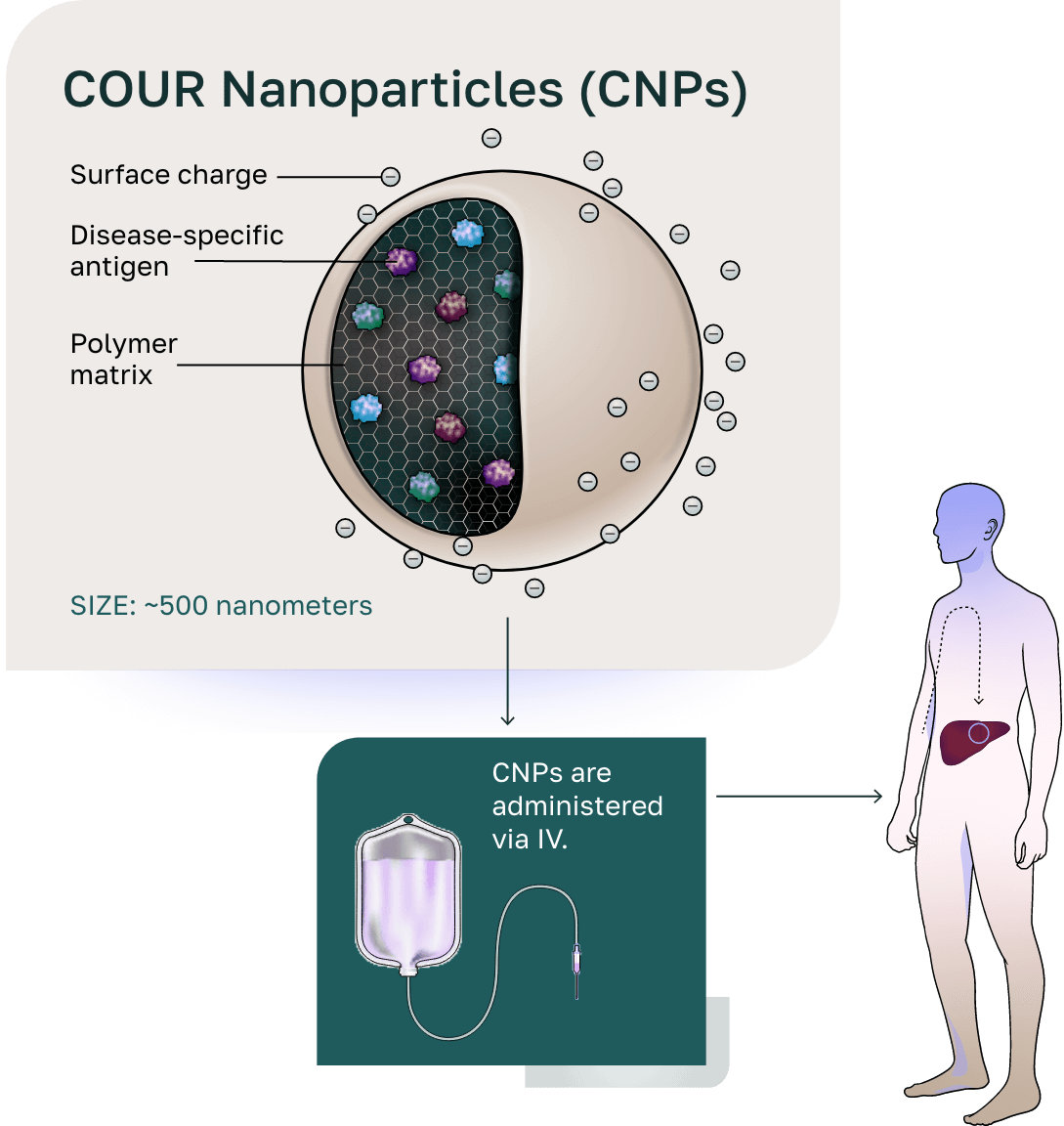
Our COUR nanoparticles (CNPs) are developed with our first-in-class, antigen-specific, immune tolerance platform technology designed to drive the discovery and development of therapies. CNPs provide safer, more targeted, and durable relief across a range of indications including primary biliary cholangitis (PBC), type 1 diabetes (T1D), myasthenia gravis (MG), and celiac disease while having applicability in as many as ~150 T-cell autoimmune diseases.
How CNPs Work
1
Administration to the patient
2
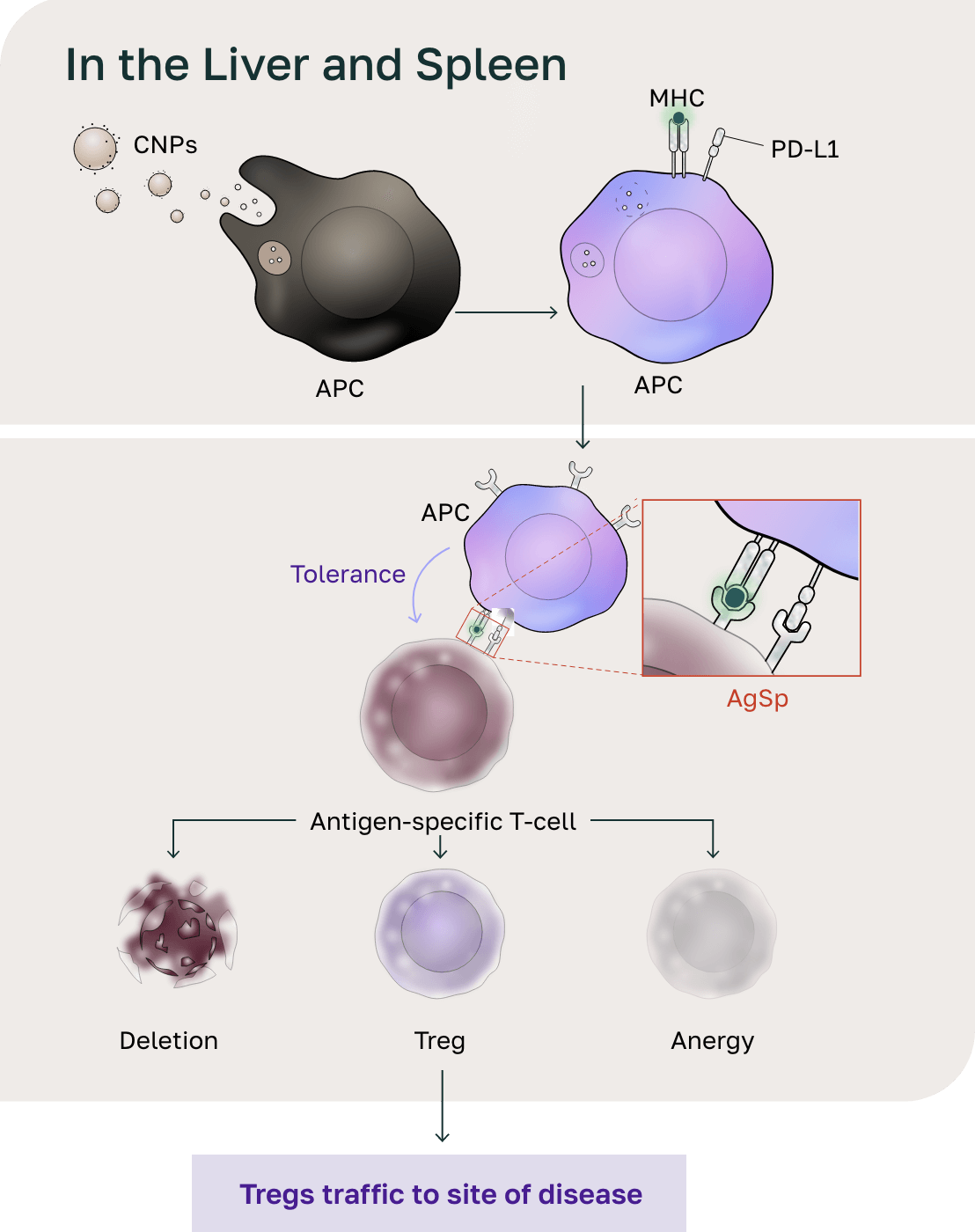
Distribution to the liver and spleen
- The size of the CNPs drives distribution to the liver and spleen and phagocytosis by antigen-presenting cells (APCs).
- Following phagocytosis, the biodegradable polymer breaks down in the phagolysosome, releasing the antigen.
- The negative charge on CNPs initiates the tolerogenic signal on APCs. The APCs present the antigens in the context of the tolerogenic signal, resulting in antigen-specific reprogramming of pathogenic T cells.
- Pathogenic T cells then undergo deletion and anergy and activate a T-regulatory (Treg) response through this endogenous mechanism for maintaining peripheral tolerance.
3
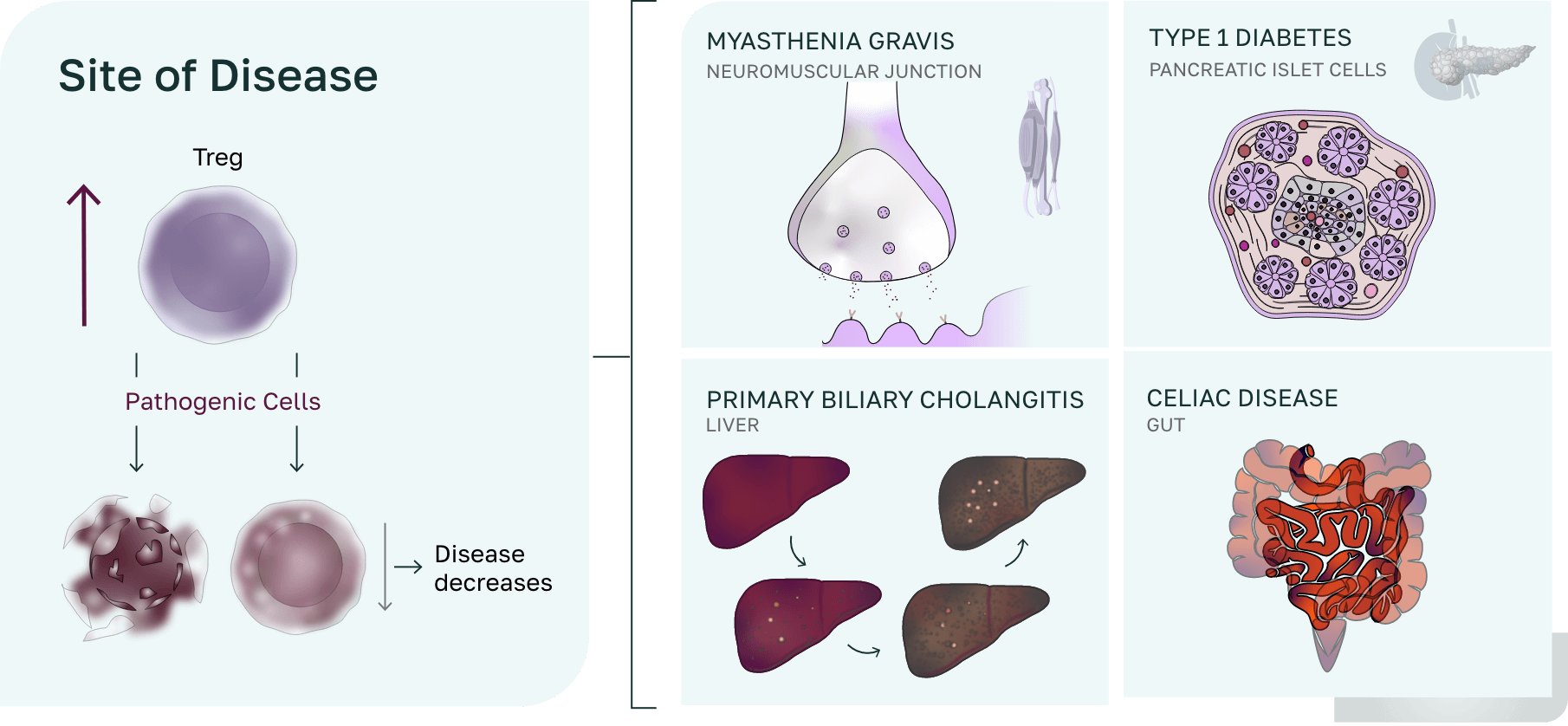
Site of disease
Pathogenic cells (CD4, CD8, B cells) are controlled through multiple mechanisms including anergy, deletion, or control by both T Regs and TR1s T cells at the site of disease, resulting in a down regulation of inflammation/infiltration and a return to homeostasis.
Manufacturing built to scale
Our robust infrastructure and strategic growth plan enable the rapid clinical advancement of our antigen-specific immune tolerance platform. With in-house expertise spanning chemistry, manufacturing, and controls (CMC) and clinical operations, we are uniquely positioned to advance novel therapies for autoimmune diseases at an unprecedented pace.
Core strengths and capabilities
In-house CMC and manufacturing
- Successfully completed 20+ Good Manufacturing Practice (GMP) human clinical trial material campaigns
- Proprietary recombinant protein manufacturing platform
Robust regulatory record
- Opened 4 investigational new drugs (INDs) in autoimmune diseases (PBC, T1D, MG, and celiac disease) with a strong regulatory track record
- Completed 2 phase 2a clinical trials and have 3 active open INDs
- Increased collaboration opportunities with the Center for Biologics Evaluation and Research (CBER) division of the United States Food and Drug Administration (FDA) due to Fast Track and Orphan Designations
Rapid Development
- Established Drug Master File (DMF) with the FDA: leverages platform toxicology and phase 1 safety data to expedite development and launch directly into proof-of-concept studies in afflicted patients
References
- National Institutes of Health. Progress in Autoimmune Disease Research. Publication No. 05-5140. U.S. Department of Health and Human Services, National Institutes of Health. March 2005. Accessed February 1, 2025. https://www.niaid.nih.gov/sites/default/files/adccfinal.pdf
- Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34(3):J168-J177. doi:10.1016/j.jaut.2009.11.018
- Cooper GS, Bynum MLK, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33(3-4):197-207. doi:10.1016/j.jaut.2009.09.008
- Fugger L, Jensen LT, Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181(1):63-80. doi:10.1016/j.cell.2020.03.007
- Rosenblum MD, Gratz IK, Paw JS, Abbas AK. Treating human autoimmunity: current practice and future prospects. Sci Transl Med. 2012;4(125):125sr1. doi:10.1126/scitranslmed.3003504
- Herold KC, Bundy BN, Long SA, et al; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613. doi:10.1056/NEJMoa1902226